this post was submitted on 29 Dec 2025
344 points (98.6% liked)
xkcd
14276 readers
190 users here now
A community for a webcomic of romance, sarcasm, math, and language.
founded 2 years ago
MODERATORS
you are viewing a single comment's thread
view the rest of the comments
view the rest of the comments
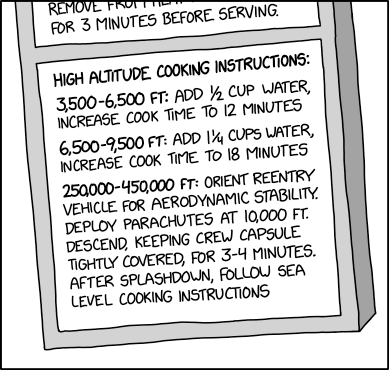
The higher altitude, the lower the atmospheric pressure, and the lower the boiling point of water. At 3300, you were down to 207°f. I used to work at a restaurant at 8k feet and we were down to a boiling point of 195, which was enough to make things like, say brownies, noticeable dryer if you didnt compensate for the extra water boiling off durrong cooking.
We actually had a fancy oven for pastry that you could set the pressure inside of, allowing us to cook things as though at sea level.
I don't know how much that is in the Boiling Water system
Meant to leave that context for for our metric friends, but my post was getting long so I shortened it. In Fahrenheit, water boils at 212°f and freezes at 32 at 1 atmosphere. (Sea level) the conversion rate is 1.8°f=°c (after subtracting those 32 'extra' degrees)
So at 207, his boiling point is only 5°f (3.33°c) lower than at sea level. Whereas where i was cooking, it was closer to 10°c - enough to considerably dry out anything baking for a decent amount of time, and throw a lot of baking chemistry off (anything leavening with baking soda, etc changes, and breads risk colapsing although that's less about the h2o, and more about the pressure iirc)
7k here, I really need to invest in a pressure cooker.